The Global Threat from the Irreversible Accumulation of Trifluoroacetic Acid (TFA)
Understanding TFA and Its Sources
TFA belongs to a subclass of per- and polyfluoroalkyl substances (PFAS) known as ultrashort-chain perfluoroalkyl acids (PFAAs). Unlike many other PFAS, TFA is exceptionally mobile and persistent in the environment. Its formation occurs when various industrial chemicals, including fluorinated refrigerants such as HFOs and HFCs, degrade in the atmosphere. This process has become more prevalent following global efforts to phase out ozone-depleting chlorofluorocarbons (CFCs) under the Montreal Protocol, leading to the adoption of alternative f-gases that ultimately contribute to TFA production.
Escalating Levels in the Environment
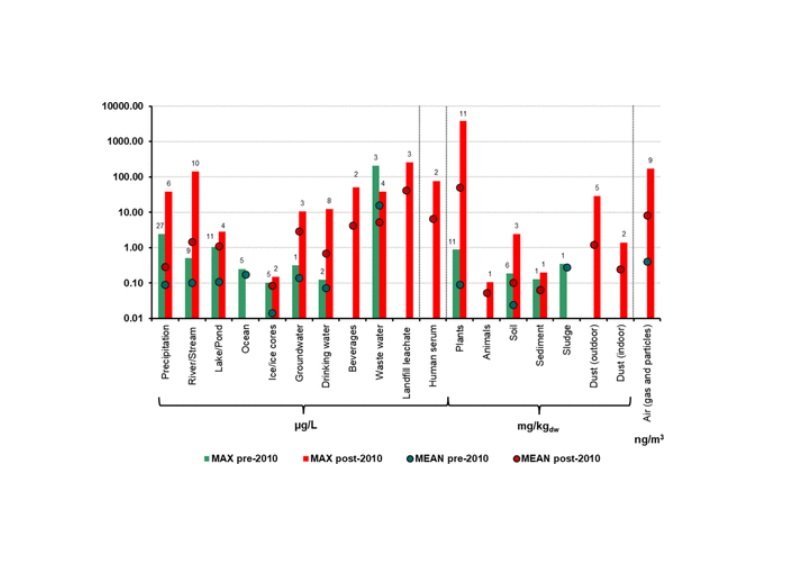
Research has shown that TFA concentrations are rising rapidly in diverse environmental media, including precipitation, groundwater, soils, plants, and even human serum. Notably, the concentrations of TFA in the environment are orders of magnitude higher than those of other PFAS. Recent studies have reported the presence of TFA in drinking water sources, with levels that frequently exceed proposed regulatory limits. For example, monitoring data from European and North American locations have documented widespread TFA contamination that is difficult to reverse due to its extreme chemical stability.
The substance is also known to bioaccumulate in crops and plant-based foods, posing a direct pathway for human and animal exposure. Data have indicated that TFA can reach significant levels in plants, including agricultural crops, through root uptake from contaminated soil and water.
Potential Health and Ecological Impacts
TFA’s potential impacts on human health and ecosystems are areas of increasing concern. Although TFA is not considered bioaccumulative in aquatic species by regulatory standards, its persistent presence in drinking water and food raises questions about chronic human exposure. Toxicological studies have suggested that TFA may exhibit liver toxicity and reproductive toxicity in mammals, although comprehensive long-term studies are still needed. Regulatory bodies in some countries have started to propose precautionary drinking water limits due to emerging evidence of its potential health risks.
The ecotoxicity of TFA, particularly for non-aquatic ecosystems, remains underexplored. Existing studies suggest that high concentrations can affect plant growth and soil quality, which may disrupt local ecosystems. Algae have been identified as particularly sensitive to TFA, with significant growth inhibition observed at certain concentrations. Additionally, due to its persistence, TFA can accumulate in terrestrial environments, potentially altering nutrient cycles and affecting biodiversity.
A Planetary Boundary Threat
The concept of a “planetary boundary” refers to limits within which humanity can safely operate without causing significant and potentially irreversible damage to the earth’s systems. TFA is considered a threat under this framework due to its persistence, mobility, and the challenge of mitigating its environmental presence. The criteria for a planetary boundary threat are met when (1) a pollutant accumulates to levels that disrupt vital earth processes, (2) those effects are global or could become global, and (3) the impacts are not easily reversible.
TFA's continuous emissions and accumulation fulfill these criteria. As emissions from industrial and consumer products continue, TFA levels are expected to increase, presenting a significant challenge to existing water treatment and environmental management practices. Its persistence means that once it enters the environment, it remains for long periods, with limited natural processes available for its breakdown.
Addressing the Threat: Policy and Innovation
To prevent further accumulation and mitigate potential irreversible impacts, global and national policy measures must be considered. This includes revisiting the use of high-volume substances that degrade into TFA and phasing out the production of TFA precursors, such as certain HFOs. Industry initiatives that align with principles of sustainable design and safer chemical alternatives are also critical in addressing this issue.
Innovation in water treatment technology could offer a partial solution, though current methods for removing TFA, such as reverse osmosis, are energy-intensive and costly. Further research into effective and economically viable treatment solutions is essential.
Conclusion
The rapid and widespread accumulation of TFA in the environment represents a potentially significant global risk. Its persistence and irreversible nature mean that the time to act is now, both through policy intervention and technological development. Protecting human health and the stability of earth’s systems requires a concerted effort to reduce emissions, phase out harmful precursors, and develop sustainable practices that prevent further environmental contamination. Only through proactive measures can future generations be safeguarded from the potential long-term impacts of TFA accumulation.